Hackensack University Medical Center Among First to Offer Genio Mask-Free Sleep Apnea Device
Multidisciplinary sleep apnea team advancing care with newly FDA-approved implant device with simplified design, fewer patient risks
December 18, 2025
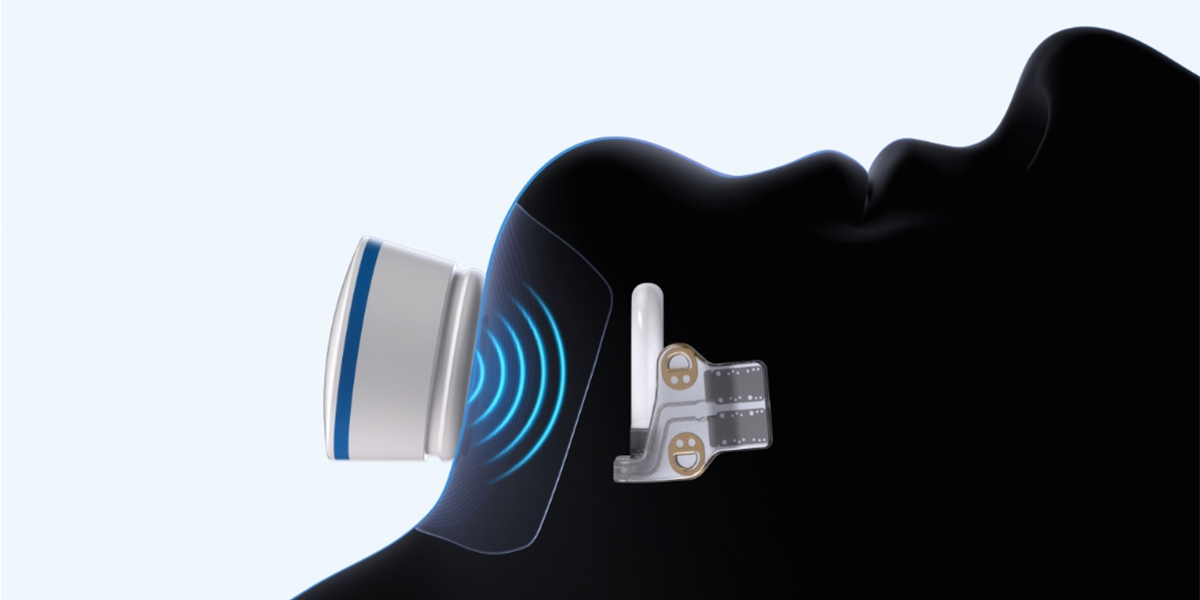
Hackensack University Medical Center will soon offer the latest leading-edge treatment for sleep apnea—a new battery-free implant device. The recently FDA-approved Genio device implants under the chin to stimulate the hypoglossal nerves, which signals the tongue muscles to contract and open the airway.
The center has a track record of embracing the latest treatments and enhancing patient comfort and compliance for this serious health issue. Hackensack University Medical Center quickly grew the largest program in New Jersey for the predecessor Inspire implantable device. A dedicated partnership with Pulmonology facilitated nuanced device current tuning, allowing growth to approximately 80 cases a year.
The Genio device is implanted through a single incision in an outpatient setting. It is powered by an external stimulator device that fits under the chin during sleep and is controlled by a smartphone app. It offers a simplified alternative to previous generation implants and a different approach for patients who cannot tolerate CPAP or BiPAP devices.
Hackensack University Medical Center Chair of Otolaryngology Brian Benson, M.D., noted, “Sleep apnea is a silent killer that is highly underdiagnosed and under treated. Patients with sleep apnea have a gigantic increase in their risk for heart attack and stroke.”
Inspire times stimulation based on tracked contraction of the patient’s chest muscles. The device has a lead wire connected to an implanted battery pack, and another wire connected to chest muscles to signal the device to stimulate throat muscles at the same time as the body activates the chest muscles to breathe. Though rare, lead wires can break, causing risk of injury. Once research determined that timing stimulation with breathing was not necessary, the simplified Genio device was conceived.
The center also treats sleep apnea with jaw appliances, jaw surgery and even a GLP-1 medication now FDA-approved for the purpose of treating sleep apnea through weight loss.
To ensure patients receive the best treatment option and in anticipation of Genio, Dr. Benson recently organized a new multidisciplinary sleep apnea conference, which will meet to review complex sleep apnea patient sleep studies and offer case discussion amongst pulmonologists, ENTs, oral surgeons and neurologists.
