Jersey Shore University Medical Center Enrolled Patients in AGENT IDE Clinical Trial
Only hospital site in N.J. to offer balloon device for in-stent restenosis following a previous coronary stenting through angioplasty
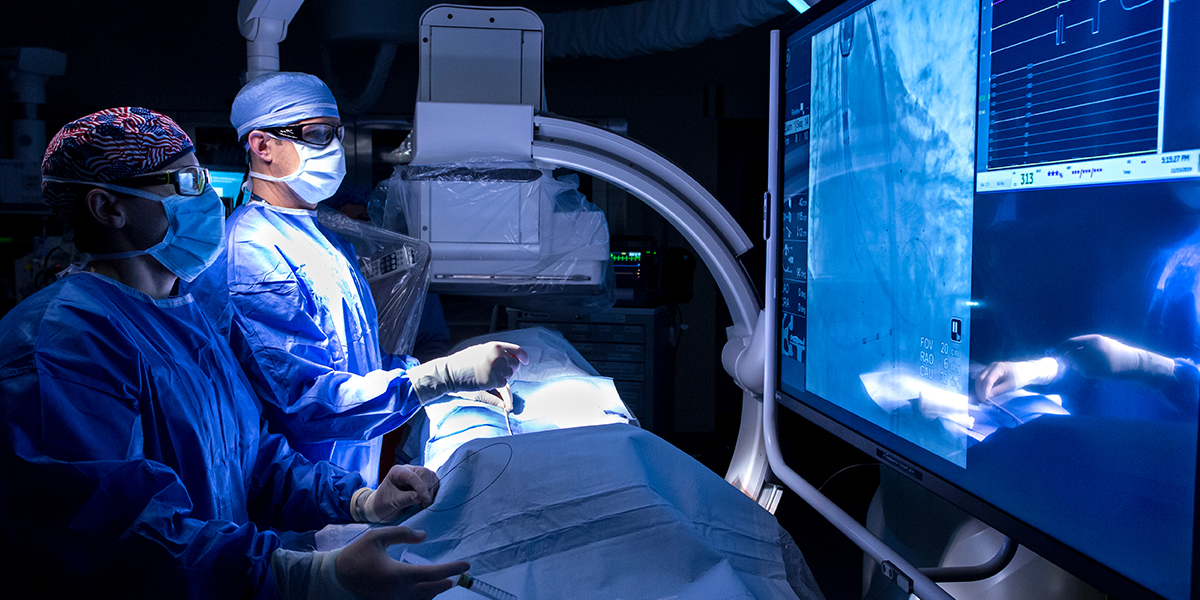
Cardiovascular experts at Hackensack Meridian Jersey Shore University Medical Center enrolled eligible patients in Boston Scientific’s AGENT IDE clinical trial to treat in-stent restenosis (ISR), following a previous implantation of a coronary stent through angioplasty. The academic medical center was the only hospital in New Jersey to participate in the trial.
ISR is currently treated by cardiologists through an additional angioplasty procedure utilizing a balloon to reopen the narrowed area again, often followed by placement of another stent. AGENT IDE is a drug-coated balloon, rather than the standard uncoated balloon, which includes a medication designed to prevent the artery from re-narrowing in the future.
“There are many circumstances where we choose to not place another layer of stent in a patient’s artery and we restore flow with a balloon only. However, future re-narrowing remains a risk. This trial will determine if the AGENT IDE balloon is more effective in addressing ISR than a standard plain balloon, ultimately providing a more personalized solution for some patients and permanently preventing recurrence of their coronary artery blockage,” says site principal investigator Matthew Saybolt, M.D. FACC, Medical Director Structural Heart Disease Program, Jersey Shore University Medical Center.
Learn more about innovative cardiovascular treatments at Jersey Shore University Medical Center.


