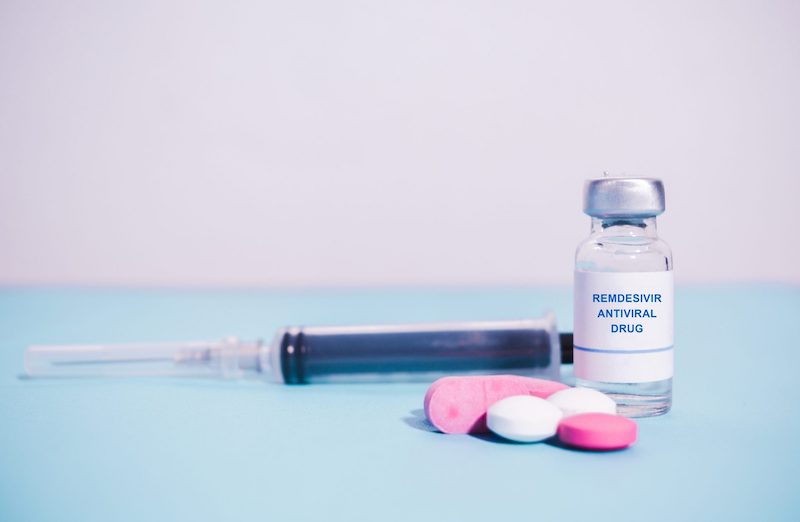
What is Remdesivir & How Is It Used For COVID-19?
May 19, 2020
Updated: 11/3/2020
Information regarding COVID-19 and vaccines are continually evolving, new details may be available since this content was developed. Please visit the CDC's website for the most up to date information.
In late October, the U.S. Food and Drug Administration (FDA) officially approved the antiviral medication remdesivir to treat patients with the novel coronavirus (COVID-19), making it the first drug to receive FDA approval for the treatment of COVID-19. The medication began making headlines this past spring as a treatment for severely ill COVID-19 patients.
Back in May, the FDA issued an emergency use authorization for remdesivir in response to preliminary results of a notable study that was released at the end of April. The study showed that hospitalized patients with severe COVID-19 who received remdesivir instead of placebo recovered 31 percent more quickly, allowing them to leave the hospital after about 11 days, instead of 15 days.
How is Remdesivir being used to fight COVID-19?
Remdesivir had never been FDA-approved to treat any condition, so the FDA had to issue an emergency use authorization to give doctors access to the medication outside of an investigational (clinical trial) setting. Beginning in May, doctors were able to give remdesivir to patients who were hospitalized with severe COVID-19, rather than limiting its usage to patients who were participating in clinical trials.
“The FDA’s Emergency Use Authorization allowed for more COVID-19 patients to receive this promising drug,” said Ihor Sawczuk, M.D., FACS, the chief research officer and president of the Northern Region for Hackensack Meridian Health.
After hospitalized COVID-19 patients nationwide began receiving remdesivir, the FDA evaluated new data about the drug’s effectiveness. In August, the FDA expanded its emergency use authorization, allowing all hospitalized patients with COVID-19 to receive the drug, including people with mild and moderate disease. And in October, the FDA changed remdesivir’s status from an emergency use authorization medication to an FDA-approved drug.
Some research shows that remdesivir doesn’t prevent death among certain patients. Additional COVID-19 treatments are still needed, but remdesivir is helping many patients recover.
What was remdesivir used for in the past?
Before its recent FDA approval and emergency use authorization, remdesivir was considered an investigational drug. It was never used to treat any conditions, but it was studied as a potential treatment for several diseases.
Originally, it was created as a possible treatment for hepatitis. In 2014, it was studied as a possible treatment for the Ebola virus. Since then, its effectiveness against other coronaviruses was studied as viruses emerged. Researchers found remdesivir to be effective against severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), although research was only conducted in test tubes and on animals, not in humans.
How is remdesivir given to COVID-19 patients?
The drug is given intravenously to hospitalized patients with COVID-19. People typically receive an injection once a day for 5 to 10 days, based on the severity of their condition.
“We took part in the remdesivir trials, and we now offer the drug as a standard of care to the COVID-19 patients admitted to our hospitals,” said Sawczuk. “When it comes to this infection, we continue to use everything at our disposal to save lives.”
How does remdesivir help patients with COVID-19?
The drug prevents the virus from producing a particular enzyme that is necessary for the virus to replicate itself. Once this happens, the virus is no longer able to spread within the body.
Research shows that when patients with moderate COVID-19 receive remdesivir, their symptoms improve more quickly. The drug has also been shown to shorten the duration of patient hospital stays. Among severely ill COVID-19 patients, remdesivir has been associated with fewer deaths.
Can anyone with COVID-19 get remdesivir?
Only hospitalized COVID-19 patients may be treated with remdesivir, whether they have mild, moderate or severe disease.
New Jersey was one of the first states to receive shipments of remdesivir after the FDA issued an emergency use authorization in May. COVID-19 patients at Hackensack Meridian Health hospitals have been treated with the medication since the earliest days of its usage as a treatment.
Next Steps & Resources:
- Meet our clinical contributor: Ihor Sawczuk, M.D., FACS
- To make an appointment with a doctor near you, call 800-822-8905 or visit our website.
The material provided through HealthU is intended to be used as general information only and should not replace the advice of your physician. Always consult your physician for individual care.
Find a doctor near me
Adjusting to a Stressful COVID-19 World

Managing COVID-19 stress? Dr. Solhkhah offers advice and support for anxiety, depression, and substance use. Find help now.
Overcoming Quarantine Anxiety

If you are feeling anxious or burnt out from COVID-related stress and isolation, you aren’t alone.
Find a doctor near me

How to Prepare for Your COVID-19 Vaccine
Prepare for your COVID-19 vaccine. Learn how to schedule your appointment, what to wear, and what to bring. Get vaccinated today.

What Are COVID-19 Vaccine Side Effects, and How Long Do They Last?
Here are answers to questions that you may have about COVID-19 vaccine side effects.
What Can You Do After You’re Fully Vaccinated Against COVID?
There are advantages to getting fully vaccinated against COVID-19.
Reduce Your Fear of Needles
A fear of needles can seem debilitating. Here are some tips to help overcome the phobia.
