Hackensack University Medical Center a Global Leader for PROTECT IV Trial Enrollment
Clinical trial evaluating long-term effectiveness of percutaneous revascularization with Impella® pump support for high-risk patients
Hackensack Meridian Hackensack University Medical Center ranks among the top enrolling sites in the world for the PROTECT IV Randomized Controlled Trial. Interventional cardiologists at the medical center were the first to treat a patient in New Jersey as part of the multicenter clinical trial and have had strong participation since enrollment began in February 2022.
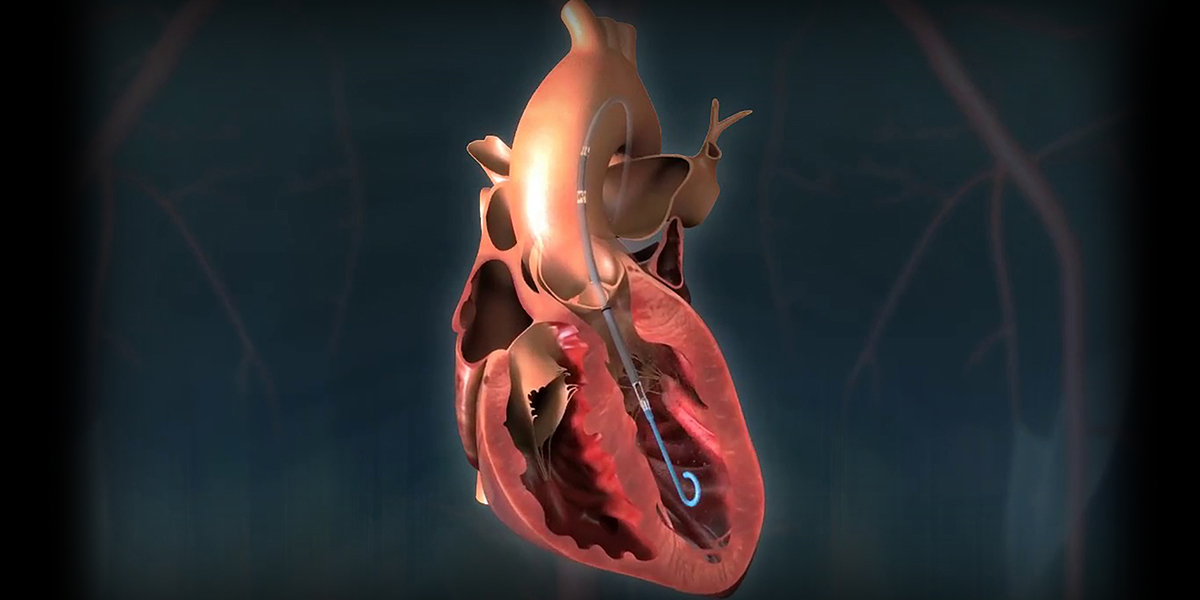
The clinical trial has been designed to evaluate the effectiveness of percutaneous revascularization supported with the Impella® pump for high-risk patients with complex heart disease and reduced heart function. The clinical trial is comparing cardiac catheterization with and without the addition of the Impella® to see if Impella® support allows better revascularization that can contribute to reduced heart disease symptoms, improved heart function, and overall health better than revascularization performed without support.
"Being a leading enroller in this cutting-edge trial is a tangible example that the cardiology program at Hackensack University Medical Center is truly at the forefront of providing exceptional top-notch care to a complex subset of cardiac patients," said Haroon Faraz, M.D., Director of Interventional Cardiology Research at Hackensack University Medical Center.
If demonstrated successfully, the Impella® may expand eligibility for catheter-based interventions to individuals with complex coronary artery disease and congestive heart failure. For these patients, physicians recently began using the Impella® device to support heart function during cardiac catheterization, inserting the device via the same catheter used during angioplasty to provide hemodynamic support to the patient during the procedure, ensuring that at no point is blood flow compromised.
While this alternative treatment for patients who are either not candidates for surgery or who have chosen not to have surgery is already applied in practice, there have not been scientifically rigorous data demonstrating that using Impella® for hemodynamic support translates into long-term clinical benefit. This is the objective of the PROTECT IV Trial.
“Being regional and national leaders in Interventional Cardiology has allowed Hackensack University Medical Center to be one of a limited number of international sites participating in this landmark clinical trial that will change the field of high risk coronary interventions,” said Ankitkumar K. Patel, M.D., Associate Director of Structural and Congenital Heart Center and Interventional Cardiologist at Hackensack University Medical Center.
“We are excited to participate in the PROTECT IV Trial and other investigation efforts that further improve our ability to care for patients with coronary artery disease. Taking the best advantage of mechanical circulatory support tools like the Impella® allows our patients to have options when more traditional therapies like coronary bypass surgery are not ideal,” said Dr. Faraz.
Dr. Patel and interventional cardiologist Pranaychandra Vaidya, M.D., treated the first patient in the PROTECT IV study at Hackensack University Medical Center. If the data show conclusively that the hemodynamic support provided by the Impella® device is superior to catheterization alone, it could lead to a new standard of care for high-risk patients with complex coronary artery disease and impaired heart function, including new guidelines from the American Heart Association and American College of Cardiology.
Learn more about innovative heart and vascular care at Hackensack University Medical Center.
